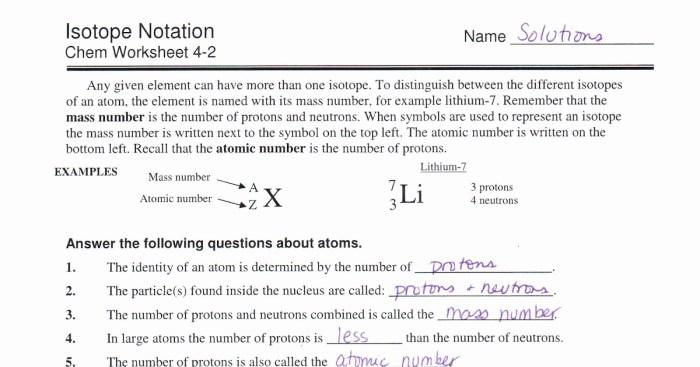
Calculate the oxidation number of sulfur in sodium metabisulfite na2s2o5 – Understanding the oxidation number of sulfur in sodium metabisulfite (Na2S2O5) is crucial for comprehending its chemical properties and applications. This compound plays a significant role in various industries, and its oxidation state directly influences its behavior.
In this comprehensive guide, we will explore the concept of oxidation numbers, the chemical structure of sodium metabisulfite, and the step-by-step process for determining the oxidation number of sulfur in this compound. We will also delve into the practical applications of sodium metabisulfite and how the oxidation state of sulfur affects its functionality.
Oxidation Number of Sulfur in Sodium Metabisulfite
In chemistry, the oxidation number of an element represents the hypothetical charge that an atom of the element would have if all of its bonds to atoms of other elements were ionic bonds. It is a useful concept for understanding the chemical reactions of elements and compounds.
Rules for Determining Oxidation Numbers
The oxidation number of an element can be determined using the following rules:
- The oxidation number of an element in its elemental form is 0.
- The oxidation number of an element in a monatomic ion is equal to the charge of the ion.
- The oxidation number of hydrogen is +1 when bonded to a nonmetal and -1 when bonded to a metal.
- The oxidation number of oxygen is -2 when bonded to a metal and -1 when bonded to a nonmetal.
- The oxidation number of a metal in a compound is equal to the charge of the metal ion.
- The sum of the oxidation numbers of all the atoms in a compound is equal to the charge of the compound.
Sodium Metabisulfite
Sodium metabisulfite is a chemical compound with the formula Na 2S 2O 5. It is a white or yellowish crystalline solid that is soluble in water. Sodium metabisulfite is used as a preservative in food and beverages, as a reducing agent in photography, and as a bleaching agent in textiles.
Oxidation Number of Sulfur in Sodium Metabisulfite, Calculate the oxidation number of sulfur in sodium metabisulfite na2s2o5
To calculate the oxidation number of sulfur in sodium metabisulfite, we can use the following steps:
- Assign an oxidation number of +1 to each of the sodium ions.
- Assign an oxidation number of
2 to each of the oxygen ions.
- Let the oxidation number of sulfur be x.
- Use the fact that the sum of the oxidation numbers of all the atoms in a compound is equal to the charge of the compound to solve for x.
The oxidation number of sulfur in sodium metabisulfite is +4.
Example Calculations
| Element | Oxidation Number | Justification |
|---|---|---|
| Sodium | +1 | Sodium is in its elemental form. |
| Oxygen | -2 | Oxygen is bonded to a metal. |
| Sulfur | +4 | The sum of the oxidation numbers of all the atoms in the compound is equal to the charge of the compound. |
Applications of Sodium Metabisulfite
Sodium metabisulfite is used in a variety of applications, including:
- As a preservative in food and beverages. Sodium metabisulfite prevents the growth of bacteria and fungi.
- As a reducing agent in photography. Sodium metabisulfite is used to develop photographic film.
- As a bleaching agent in textiles. Sodium metabisulfite is used to bleach fabrics.
Questions and Answers: Calculate The Oxidation Number Of Sulfur In Sodium Metabisulfite Na2s2o5
What is the oxidation number of sulfur in sodium metabisulfite?
The oxidation number of sulfur in Na2S2O5 is +4.
How do you calculate the oxidation number of sulfur in sodium metabisulfite?
To calculate the oxidation number of sulfur in Na2S2O5, assign oxidation numbers to the other elements based on their common oxidation states and then solve for the oxidation number of sulfur.
What are the applications of sodium metabisulfite?
Sodium metabisulfite is used as a preservative in food and beverages, an antioxidant in pharmaceuticals, and a bleaching agent in the textile industry.